Please, activate Compare option to use this widget.
Need help? Call us:
+91 97147 40045
Shopping cart (0)
Subtotal: $0.00
Spend $3,050.00 to get free shipping
Congratulations! You've got free shipping.
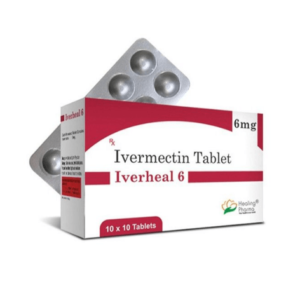
Ivermectin 6mg (Iverheal)
$70.00 – $260.00


Ivrea Cream (Ivermectin)
$15.00 – $60.00
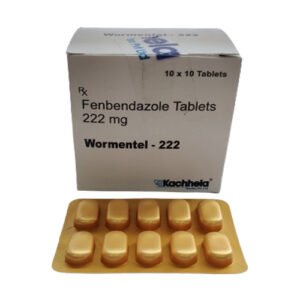
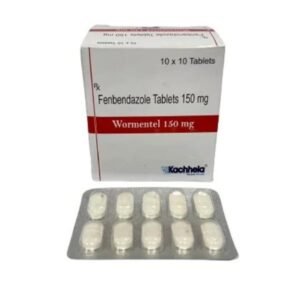
Fenbendazole 150 Mg
Brand:
33 people are viewing this product right now
$60.00 – $340.00
Shipping calculated at checkout.
| Fenbendazole 150 Mg | |||
|---|---|---|---|
| Pack Size | Price | Per Unit | Qty |
| 60 Tablets | $60.00 | $1.00 | |
| 100 Tablets | $90.00 | $0.90 | |
| 200 Tablets | $140.00 | $0.70 | |
| 300 Tablets | $208.00 | $0.69 | |
| 500 Tablets | $340.00 | $0.68 | |
🔥 Buy More Save More!
Buy 3 items get 5% OFF
on each productBuy 6 items get 10% OFF
on each productBuy 10 items get 15% OFF
on each product
Estimated delivery:5 days
SKU:
Fenbendazole 150 mg
Categories: Anti Worm
Have any Questions?
Feel free to Get in touch
Guarantee Safe and Secure Payment Checkout
Fenbendazole 150 mg – Product Description
Brand Name: [Specify Brand Name]
Active Ingredient: Fenbendazole 150 mg
Drug Class: Benzimidazole Anthelmintic
Manufactured By: Kachhela Ltd.
Packing Details: strips of 10 dispersible tablets per pack.
Description:
Fenbendazole 150 mg is a broad-spectrum anthelmintic used for the treatment of parasitic infections in animals. It works by disrupting the metabolism of parasites, leading to their elimination from the body. It is commonly used in veterinary medicine for dogs, cats, cattle, horses, and other animals.
Uses:
Fenbendazole 150 mg is prescribed for the treatment and control of:
✔ Gastrointestinal Worms – Roundworms, Hookworms, Whipworms
✔ Lungworms
✔ Tapeworms (Taenia spp.)
✔ Giardia (Protozoal Infection in Dogs & Cats)
✔ Parasitic Infections in Cattle, Horses, and Poultry
Dosage & Administration:
-
Dogs & Cats: 50 mg per kg body weight once daily for 3–5 days, or as directed by a veterinarian.
-
Cattle & Horses: Dosage varies depending on weight and type of parasite.
-
Route of Administration: Oral – Can be given directly or mixed with food.
-
Consult a veterinarian for exact dosage adjustments based on the animal’s weight and condition.
Side Effects:
Common side effects may include:
✔ Mild diarrhea
✔ Vomiting
✔ Loss of appetite
Serious side effects (rare):
⚠ Allergic reactions (swelling, itching, difficulty breathing)
⚠ Severe vomiting or diarrhea (if overdosed)
Precautions & Warnings:
⚠ For veterinary use only—not intended for human consumption.
⚠ Ensure the correct dosage based on the animal’s weight and species.
⚠ Not recommended for pregnant animals unless advised by a veterinarian.
⚠ May interact with certain anthelmintics or medications—consult a vet before use.
Storage Instructions:
✔ Store at room temperature (below 30°C), away from moisture and direct sunlight.
✔ Keep out of reach of children and animals.
Disclaimer:
This information is for educational purposes only. Always consult a veterinarian before using Fenbendazole 150 mg.
Be the first to review “Fenbendazole 150 Mg” Cancel reply
Related products
You may add any content here from XStore Control Panel->Sales booster->Request a quote->Ask a question notification
At sem a enim eu vulputate nullam convallis Iaculis vitae odio faucibus adipiscing urna.






Reviews
There are no reviews yet.